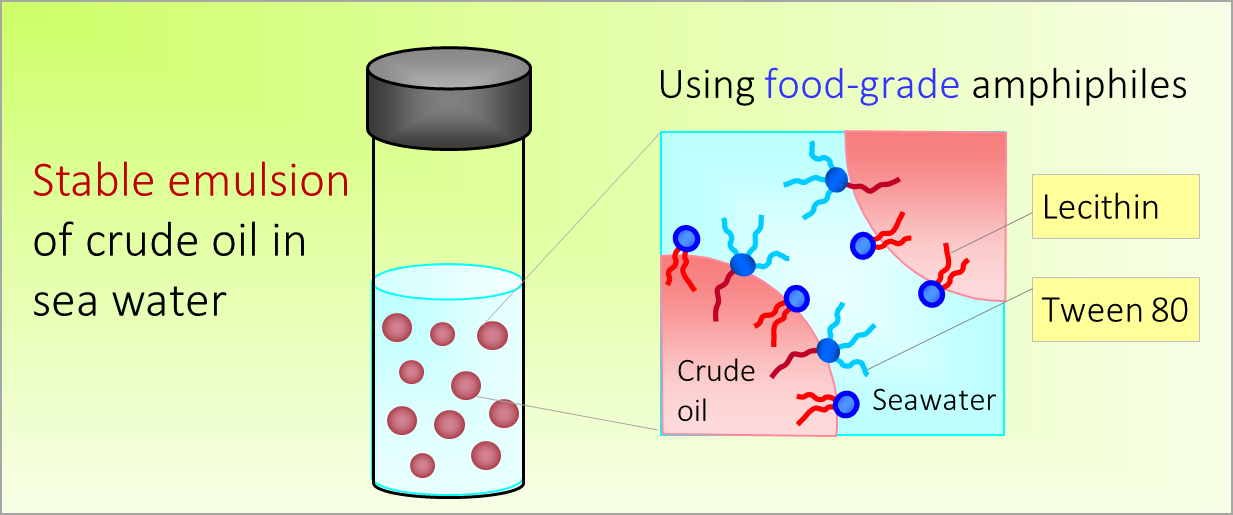
What We Do
We create and invent new materials with unusual or exceptional properties. Read an interview with Prof. Raghavan…

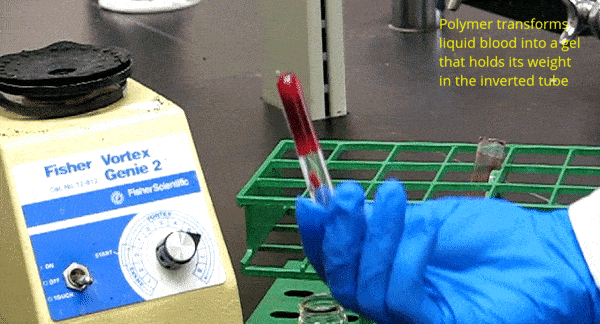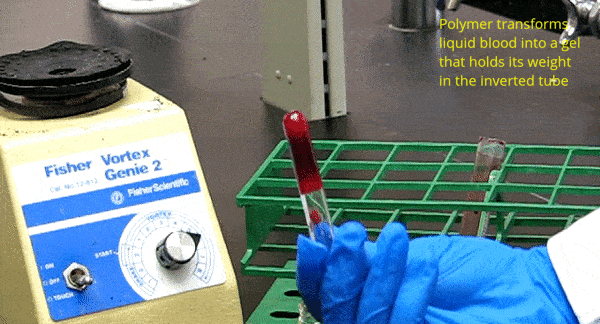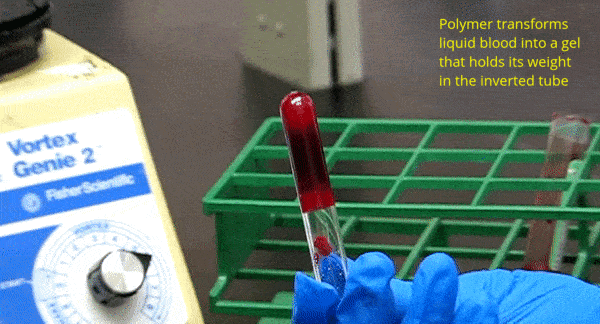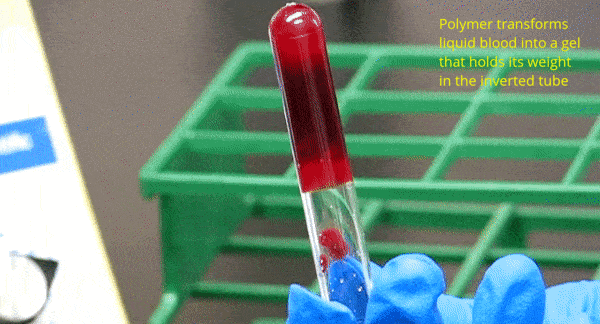
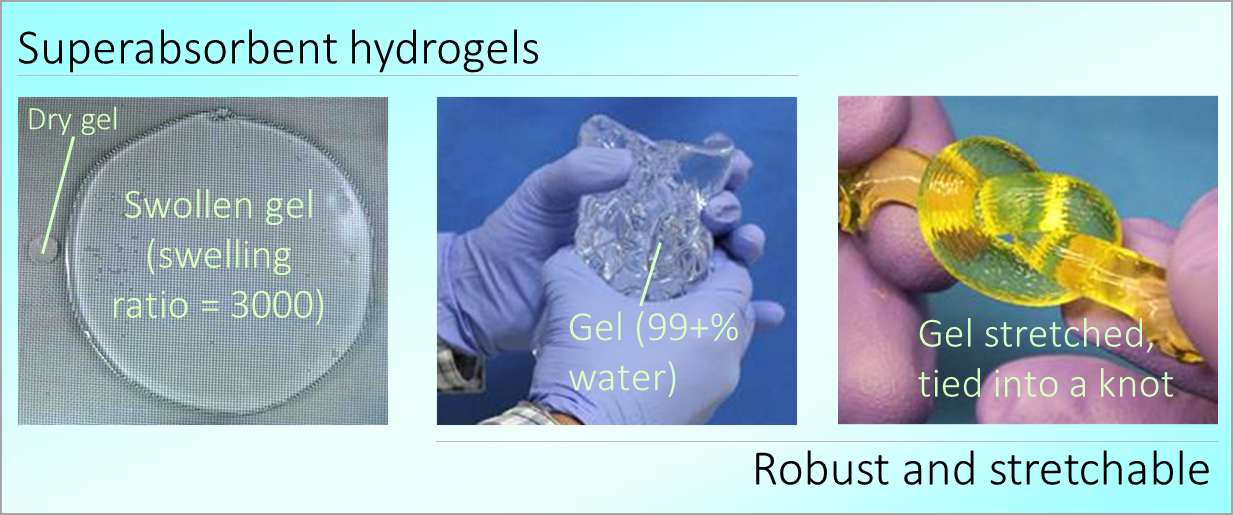
The materials we create are usually soft solids or viscous fluids. We try to tailor their mechanical and flow properties. More…
Our specialty is “smart” materials, whose properties can be switched (by light, heat, electricity, etc.). More…

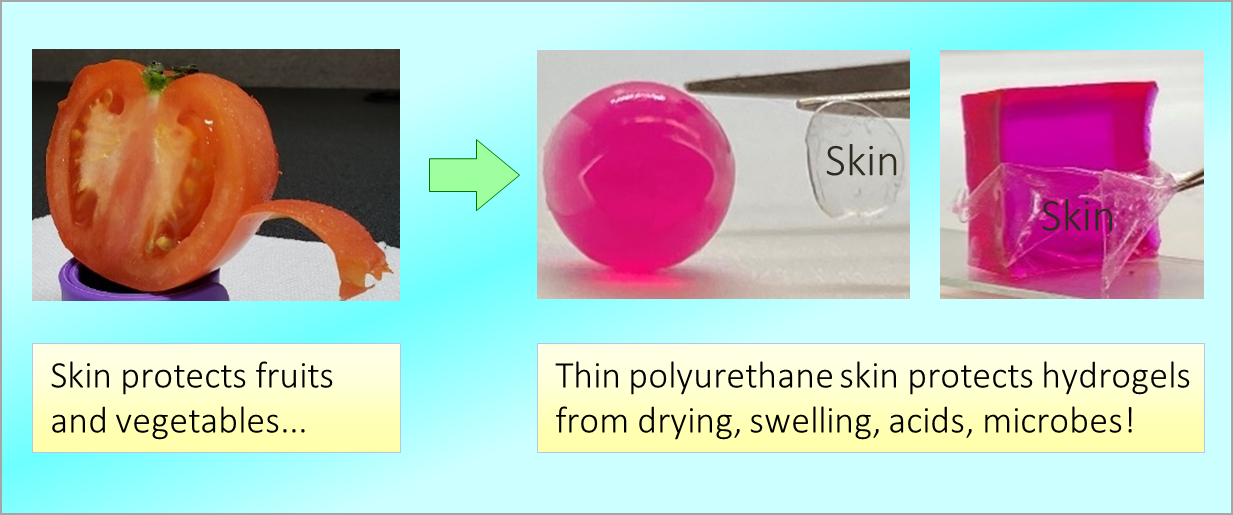
Our inventions often draw inspiration from nature at various length scales (macro, micro, nano). More…

We emphasize simplicity in our work. That is, we try to find simple routes to new materials using cheap ingredients. More…

Our scientific focus is on discovering the rules for molecular self-assembly into various nanoscale structures. More…
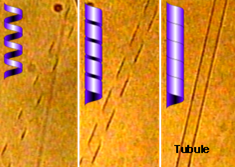
Techniques in which we have expertise include rheology, light scattering, and neutron scattering (SANS). More…